Siri Dominguez
2/16/2014
F Block
Yeast Cell Respiration Lab
Abstract: In this lab, we tested how the use of sucrose, glucose, protein, and starch affected the cell respiration of yeast. We tested this by creating similar test tubes that contained different substances, and then initiated the process by adding the elements needed for cell respiration to begin. We found that our test tubes containing sucrose and glucose created the most gas, while our test tubes containing starch and protein yielded hardly any gas. From this we concluded that cell respiration requires glucose in some form in order to successfully initiate cell respiration.
Introduction: Cellular respiration allows organisms to use (release) energy stored in the chemical bonds of glucose (C6H12O6). The energy in glucose is used to produce ATP. Cells use ATP to supply their energy needs. Cellular respiration is therefore a process in which the energy in glucose is transferred to ATP (faculty.clintoncc.suny.edu). In class we studied how yeast uses this equation: 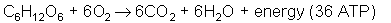
for cell respiration since it can not perform photosynthesis. This helped us design our experiment and produce our hypothesis.
Hypothesis: If we add 1g of any substance that does not contain glucose to our test tubes, then the yeast will not be able to produce ATP, which would be represented by the amount of gas produced by the test tube.
Materials:
- 4 test tubes (vials)
- 1g of sucrose
- 1g of pure glucose
- 1g of starch
- 1g of protein
- 1g of yeast (each)
- 35 mL of warm water (each)
- .1g salt (each)
- 4 rubber stoppers with tube system used to measure the gas produced
- timer
- scale
Procedure:
- Put 1g of yeast, .1g of salt, and 35 mL of warm water into each test tube
- Add specific ingredients to corresponding test tube (sucrose, glucose, starch, and protein)
- shake until contents are dissolved
- let all test tubes sit for 5 min
- insert rubber stopper and take starting measurement
- in increments of 1 min, take measurements for each test tube
- stop after 5 min
Results:
Substances
|
Baseline
|
1min
|
2min
|
3min
|
4min
|
5min
|
Sucrose
|
1.6 mL
|
2.2 mL
|
2.8 mL
|
3.4 mL
|
5.8 mL
|
6.4 mL
|
Glucose
|
.6 mL
|
.6 mL
|
.8 mL
|
1 mL
|
1 mL
|
1.2 mL
|
Protein
|
1 mL
|
1 mL
|
1 mL
|
1 mL
|
1 mL
|
1 mL
|
Starch
|
1.6 mL
|
1.6 mL
|
1.6 mL
|
1.6 mL
|
1.6 mL
|
1.6 mL
|
Conclusion: After reviewing our findings, we concluded that our hypothesis was correct. In comparison with our control, the test tube that contained sucrose, our results depicted what we predicted in our hypothesis; the test tubes that did not contain any form of glucose did not produce much gas (CO2). In our experiment we had three constants, The amount of yeast, water, and salt added to each test tube. With these constants, we hoped to increase our chances of eliminating any outside factors that might affect our experiment. However, we found that our control produced a lot more gas than the other products. Glucose should have produced just as much CO2 as our control, sucrose. This finding might have been due to incorrect measurements made when mixing the different substances together, or possible outside contamination left over in the test tube by other substances.
Citation:
faculty.clintoncc.suny.edu
No comments:
Post a Comment